Osteoarthritis Research
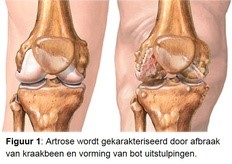
Osteoarthritis (Figure 1) is the most common chronic disease in our country. As many as 1.2 million people have osteoarthritis and struggle daily with the pain and movement limitations that osteoarthritis entails. In addition to joint replacement surgery in the end stage of the disease, there is no effective drug. Patients are therefore dependent on physiotherapy and palliative care for years. Risk factors of osteoarthritis are age, obesity, mechanical overload and hereditary predisposition.
What’s the problem?
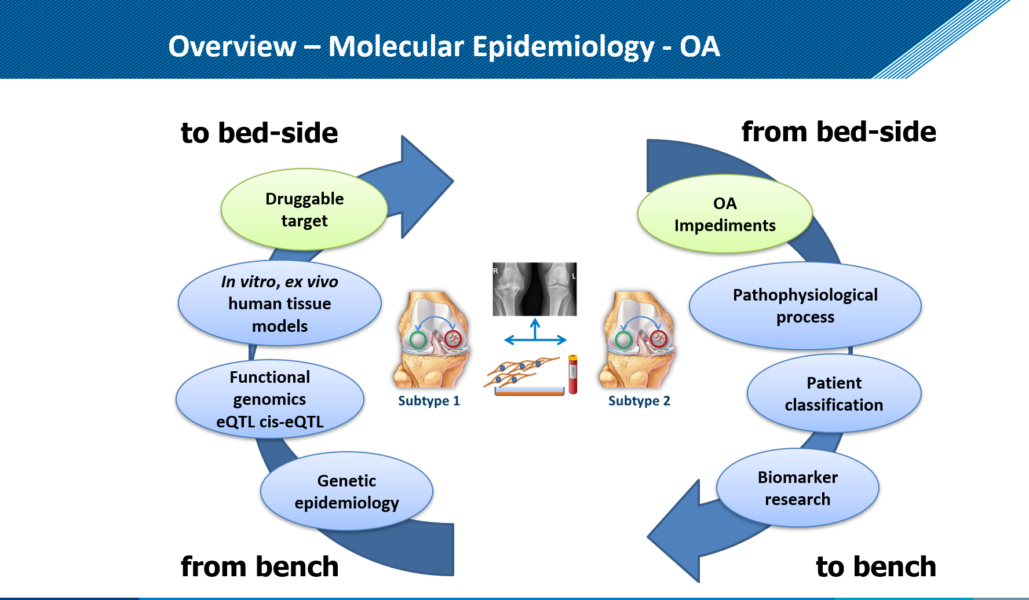
An important problem that hinders the development of medicines is that the pharmaceutical industry has until now been looking for a medicine for the average osteoarthritis patient. But it doesn’t exist. Osteoarthritis is caused by various causes (eg hereditary predisposition, overload, overweight) and the disease manifests itself in different ways. Which patient has which form of osteoarthritis has not yet been properly investigated and is difficult to recognize. Not even with the help of modern equipment like the MRI. The different disease processes arise because the cells in the joint react differently to damage to the tissue.
The RAAK study
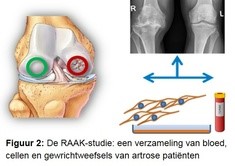
In the research we use the RAAK study (Figure 2). In this study, we collected blood and joint tissues from osteoarthritis patients during joint replacement surgery. The tissues must be removed in order to place the artificial joint. Because the joint tissues (bone, cartilage, mucosal layer) are collected immediately after surgery, we can also remove living cells from the tissues.
The research
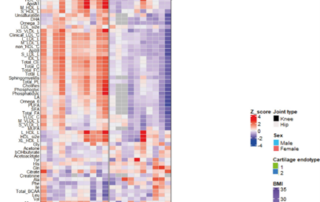
Mapping diversity We want to map the diversity of the molecular pathways, or disease processes, that lead to osteoarthritis and thus distinguish different patient groups from each other. We therefore study in the joint tissues, the hereditary make-up, the activity of properties (genes) and the mechanism of cells to control the activity of these properties.
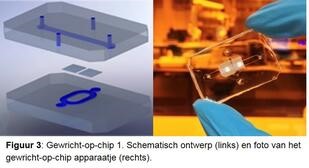
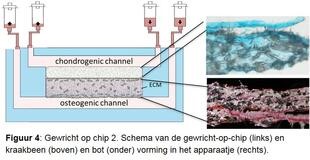
Investigate osteoarthritis disease process in detail. In order to study the various disease processes in detail, we are developing a joint-on-a-chip together with Eindhoven University of Technology (Figures 3 and 4). This is a tiny device that has two connected spaces. One is filled with cartilage cells, the other with bone cells from the RAAK study, and new cartilage and bone tissue is created in the device using growth factors. Because we expose the cells to mechanical stress, for example by hitting the cells with tiny hammers, we can mimic the osteoarthritis process. We also change the genetic makeup of the cells to investigate patient-specific causes. By accurately determining where things go wrong during osteoarthritis in different patients, we also get leads for the development of (new) medicines. We also call this tailor-made treatment.
Signaling molecules to observe disease process
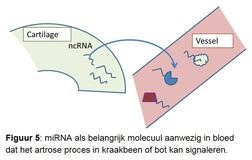
There is no adequate diagnostics available that can observe and track the precise osteoarthritis process in the joint tissues over time. This poses major problems when testing new drugs. This is because it is not possible to select patients who, on the basis of the disease process present, will probably benefit most from the specific target point of the developed drug. The research is therefore aimed at using a promising new molecular signaling molecule. These are so-called micro-RNAs. Micro-RNAs are small pieces of genetic material that regulate and control gene activity and thus specific processes in tissues (Figure 5). What is unique is that micro-RNAs also ensure the exchange of messages about the condition of the tissues in the body via the bloodstream. This is when we can intercept the micro-RNAs and their message and use it as a biomarker.
Most Recent Publications Osteoarthritis
New publication shows blood biomarkers for senescence endotypes in osteoarthritis patients
When cells reach a permanent growth arrest (Senescence) and resist death, this can result in numerous diseases, such as osteoarthritis. Here we show the presence of two different types of senescence in the blood metabolome of osteoarthritis patients. These blood metabolome patterns could function [...]
The role of TNFRSF11B in development of osteoarthritic cartilage.
The role of TNFRSF11B in development of osteoarthritic cartilage. Rheumatology (Oxford) | 2022 Feb 2;61(2):856-864. | doi: 10.1093/rheumatology/keab440 Rodríguez Ruiz A, Tuerlings M, Das A, Coutinho de Almeida R, Suchiman HED, Nelissen RGHH, Ramos YFM, Meulenbelt I. Abstract Objectives: OA is a complex genetic disease [...]
High-impact FN1 mutation decreases chondrogenic potential and affects cartilage deposition via decreased binding to collagen type II
High-impact FN1 mutation decreases chondrogenic potential and affects cartilage deposition via decreased binding to collagen type II I| November 5 - 2021 | Science Advances Marcella van Hoolwerff, Alejandro Rodríguez Ruiz, Marga Bouma, H. Eka D. Suchiman, Roman i. Koning, Carolina r. Jost, Aat a. Mulder, [...]
OSTEOARTHRITIS TEAM